Technology
Novel Monoclonal Antibodies for Immune and Inflammatory Diseases
We are advancing first-in-class therapies, including EB05 (Paridiprubart), that target critical points in inflammatory and innate immune response pathways. Our goal is to transform the lives of patients suffering from acute and chronic diseases.
First in Class mAb
Our most advanced drug candidate is EB05 (Paridiprubart), a monoclonal antibody developed for acute and chronic disease indications that involve dysregulated innate immunity responses.
Well Documented Mechanism of Action
EB05 (Paridiprubart) dampens TLR4 signaling by blocking receptor dimerization and has been shown to be independent of ligand type and concentration.
Fast Tracked by FDA
Without EB05 (Paridiprubart)
With EB05 (Paridiprubart)
Without EB05 (Paridiprubart)
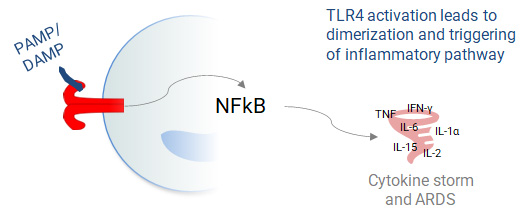
With EB05 (Paridiprubart)
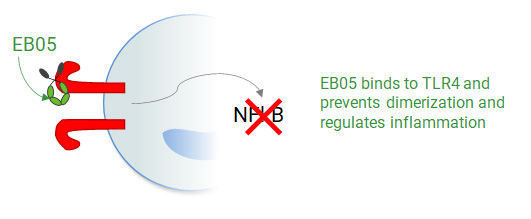
Acute Respiratory Distress Syndrome (ARDS)
A Leading Cause of ICU Admissions Globally
Life-threatening form of respiratory failure
- Exaggerated immune response
- Inflammation and injury to the lungs
- Edema that deprives the body of oxygen
Causes
- Infections by virus (i.e. Covid-19, influenza) or bacteria, smoke/chemical inhalation and chest injury
Standard of Care
- Few meaningful treatments, other than supplemental oxygen and mechanical ventilation
- Substantial evidence that multiple causes of ARDS are mediated by the TLR4 pathway
ARDS – A Significant Disease Burden
Annual Cases Globally
%
ICU Mortality Rate
$ Cost Per Patient (Average U.S.)
Sars-CoV2 and Other Pathogens
Ageing Population
Co-morbidities and Risk Factors
EB05 (Paridiprubart) Decreases Mortality by 84% in Critically Severe Patients
Phase 2 Results
Statistically Significant Mortality Trend in Critical Patients
28-Day Mortality Rate by Treatment Group*
n=33; p=0.04
“Achieving statistical significance in the most difficult-to-treat patients has increased our excitement and belief that Paridiprubart could become a standard-of-care treatment option.”
Critically ill patient population*
- 28-day death rate of 7.7% (1/13) in the EB05 (Paridiprubart) arm vs. 40.0% (8/20) in the placebo arm
- 84% reduction in the risk of dying (HR: 6.12 placebo vs. EB05 (Paridiprubart); 95% CI: 0.77-49.06; p=0.088).
- All patients received Standard of Care (SOC): ~85% received dexamethasone (or other steroids); >40% received both tocilizumab and a steroid; well balanced
Favorable Safety Profile
- Paridiprubart, an anti-TLR4 antibody candidate, has been evaluated in multiple clinical studies (with 600+ subjects in total)
*World Health Organization’s Covid-19 Severity Scale Level 7 critically severe patients, receiving extracorporeal membrane oxygenation (ECMO), invasive mechanical ventilation plus organ support, or both. ITT= Intend to Treat (i.e., no randomized subjects were excluded).
EB01- A Topical Daniluromer Formulation for Allergic Contact Dermatitis

First-in-Class Anti-Inflammatory
EB01 (daniluromer) is designed to inhibit the inflammatory process at its inception rather than after inflammation has occurred.
Alternative to Steroids
Broad Potential Opportunities
Daniluromer’s Novel Mechanism of Action
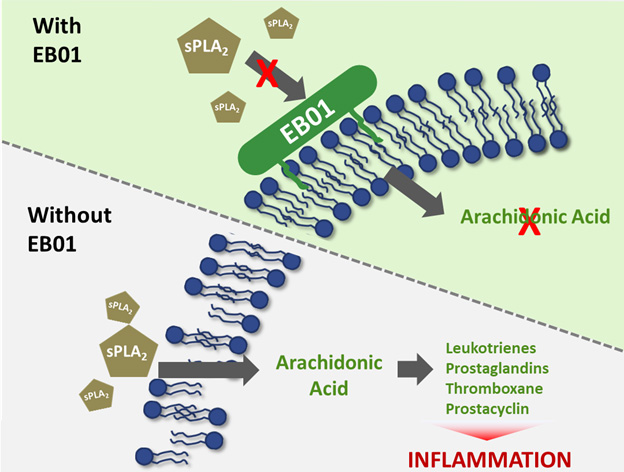
sPLA2 inhibitors represent a novel approach treating inflammation without the safety concerns of current therapies
Upstream Modulation
Daniluromer is a new chemical entity that exerts its anti-inflammatory activity through the inhibition of certain pro-inflammatory enzymes known as secretory phospholipase 2, or sPLA2.
Efficacy and Durability
EB01 (daniluromer) has demonstrated efficacy for the treatment of ACD in multiple clinical trials.
Well Tolerated
Daniluromer preserves cell membrane integrity leading to a better safety profile.
Daniluromer inhibits sPLA2 from degrading phospholipids to produce arachidonic acid. Arachidonic acid is processed via the LOX-COX pathway to produce several pro-inflammatory signaling molecules. Daniluromer exerts its anti-inflammatory activity upstream of currently approved NSAIDs, which target the LOX-COX pathway.
Allergic Contact Dermatits (ACD)
The Leading Occupational Health Issue Related to Dermatology
Type IV, or Delayed-Type, Hypersensitivity Reaction
- Immune system sensitized following initial contact with allergen
- Subsequent contact results in cell-mediated allergic response at the point of contact
- Often highly visible on face and hands
Standard of Care
- No labelled products available
- Corticosteroids cannot be used for prolonged periods due to safety concerns
Few Treatment Options for Chronic ACD Patients
Known Contact Allergens
%
Unable to Identify Causal Allergen
No Known Labelled Drugs
ACD Adversely Impacts the Workplace
- Loss of Productivity
- Complexitiy of Mitigation
- Lost Income and Disability Claims
Corticosteroids and Immunomodulators
- Safety Concerns
- Side Effects
Physicians strongly desire additional treatment options**
“ACD…can make you quit your job.”
“Maybe topical steroids help a little but I almost never use them”
“Topicals are easier to use and they are a safer option than oral medications.”
1.0% EB01 Met Primary and Secondary Endpoints with Statistical Significance
Phase 2B – Preliminary Topline Results of EB01
For the treatment of chronic moderate-to-severe allergic contact dermatitis
Total CDSI Score*
Mean Percent Improvement from Baseline
Total ISGA Score*
% Patients with a ≥2 Point Improvement from Baseline
“We are pleased that the study findings demonstrated that the 1.0% EB01 cream helped patients with moderate-to-severe disease significantly reduce their symptoms and achieve clear or almost clear skin in more than half the cases. A significant improvement was evident as early as two weeks from initiation of treatment.”
Efficacy
- 1.0% EB01-treated patients demonstrated a relative improvement of >50% (CDSI) and >80% (ISGA) over placebo/vehicle
Response at 15 Days (CDSI)
- 44% for 1.0% EB01 vs. 29% for placebo; p=0.05
Response at Follow Up (CDSI)
- 64% for 1.0% EB01 vs. 44% for placebo; p=0.04
Lowest Efficacious Dose
- 1.0% EB01 was identified as the lowest efficacious dose over 2.0% and 0.2%
Safety
- No serious treatment-related adverse events were reported across all doses
*Intention to Treat (ITT) population; statistical analysis based on last observation carried forward (LOCF). Placebo (n=84); 1.0% EB01 Cream (n=19). Contact Dermatitis Severity Index (CDSI) at Day 29. Success on the Investigator’s Static Global Assessment (ISGA) is defined as a 2-point reduction and score indicating clear/almost-clear skin. Topline study data are preliminary and subject to change.
** Company sponsored research, including interviews of Key Opinion Leaders
Further Reading
Imai et al., 2008: This study looked at the role of TLR4 in ALI. Mice deficient in TLR4 were resistant to acid-induced ALI and while H5N1 influenza rapidly induced ALI in wild-type mice, TLR4 deficient mice were resistant to H5N1-induced ALI, suggesting a causative role for TLR4 in
Shirey et al., 2016: In this study, an anti-TLR4 antibody protected mice from lethal influenza
Perrin-Cocon et al., 2017: A novel small molecule TLR4 antagonist (FP7) was tested in an in vivo mouse model of influenza. FP7 blocked TLR4 stimulation and protected mice from influenza-induced lethality and reduced inflammatory cytokine expression and
Zhou et al., 2018: An anti-TLR4 monoclonal antibody was studied in a rat model of ARDS. The rats treated with the anti-TLR4 antibody showed lower respiratory frequency, lung permeability, lung edema, inflammatory infiltration, and tumor necrosis factor and interleukin expression levels in lungs along with lower TLR4, TLR9, MyD88, and nuclear factor expression in macrophages.
Domitrovic 2018: TLR4 monoclonal antibodies were evaluated both in vitro and in a rat model of ARDS. Stimulating macrophages with TNF-alpha along with anti-TLR4 antibody eliminated the upregulation and secretion of cytokines. Pre-treating rats with anti-TLR4 antibody prior to ventilation.
Wang et al., 2013: This study showed that patients suffering from ARDS caused by H1N1 infection had significantly elevated levels of CXCL10 in their serum compared to a control group. An anti-CXCL10 monoclonal antibody increased survival time, reduced lung edema, and significantly decreased ALI in a mouse model of H1N1 infection.
Ichikawa et al., 2013: In this study, ARDS was induced in mice by both non-viral and viral means. Mice deficient in CXCL10 or CXCR3 had improved severity and survival of both viral and non-viral ARDS.
Lang et al., 2017: This study explored the role of CXCL10 in a rat model of LPS-induced ARDS. Expression of CXCL10 and CXCR3 increased after LPS-induction. An anti-CXCL10 antibody decreased the severity of ARDS.